Paper Clip Activity: Unit 1
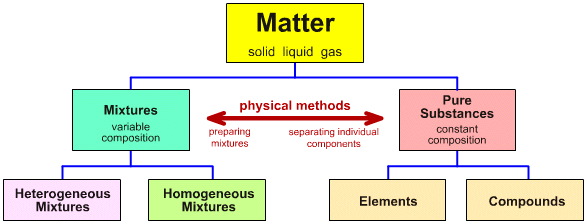
Today in class, we started out by taking notes on the Particulate Nature of Matter section. We first learned about monatomic, diatomic, and polyatomic elements. Monatomic elements exist as single atoms, diatomic elements consist of two of the same atoms bonded together, and polyatomic elements consist of the same kind of atoms bonded in groups of three or four. After these elements, we moved on to learn about compounds. Compounds consist of two different kinds of atoms chemically bonded together. For example, carbon dioxide consists of one carbon atom bonded with two oxygen atoms. Everything so far has been a pure substance because they have not been mixed with any other types of elements or compounds. After this we learned about mixtures, which are not pure substances. There are two different types of mixtures, homogeneous and heterogeneous. In a homogeneous mixture all parts have to same makeup. For example coffee mixed with sugar is a homogeneous mixture. Any solution is homogeneous. In a heterogeneous mixture, there may be more of a certain substance in one part than in a different part. For example if you take two handfuls of skittles, you may have more green skittles in your right hand than in your left hand. After these notes, we moved on to the paper clip activity.
Paper Clip Activity
After we took the notes, we began our paper clip activity. In this activity we used different sized paper clips to represent different atoms. We used the symbols Jb for jumbo, Sm for small, and Cl for colored. Then we were given 8 different chemical formulas and we had to make them using the paper clips. For example Sm3 (subscript), then we would attach three small paper clips together. Then we answered the number of atoms in the sample and the number of molecules in the sample. In this case it was 3 and 1. After we finished we made up a couple of our own. This activity taught us about atoms, elements, compounds, molecules, and mixtures. We used what we learned in the notes to help us with this activity.
This would be an emample of Jb3+Jb.
The next scribe will be Jane B.